In the vast domain of chemistry, ions are integral components that contribute to the diverse and dynamic nature of matter. At their core, ions are charged particles formed when atoms gain or lose electrons, resulting in an imbalance of positive or negative charge. This fundamental definition sets the stage for understanding the multifaceted roles that ions play in chemical reactions, biological processes, and everyday phenomena. Now, let's delve deeper into the world of ions, exploring the intricacies of positive ions, negative ions, and polyatomic ions.
Positive Ions: Cations
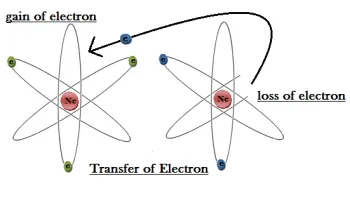
Positive ions, also known as cations, emerge when atoms
undergo a process called ionization, wherein they lose one or more electrons.
This loss of negatively charged electrons leaves behind a positively charged
particle, creating an imbalance of charge within the atom. Cations exhibit a
net positive charge and are attracted to negatively charged particles or
regions.
These charged particles play pivotal roles in various chemical reactions and biological processes. For instance, sodium ions (Na⁺) and potassium ions (K⁺) are essential for nerve conduction and muscle contraction in the human body. Similarly, calcium ions (Ca²⁺) contribute to bone strength and cellular signaling. The prevalence of cations underscores their significance in maintaining physiological functions and sustaining life.
Negative Ions: Anions
Conversely, negative ions, or anions, are formed when atoms
gain one or more electrons through the process of ionization. This gain of
negatively charged electrons results in an excess of negative charge within the
atom, giving rise to a net negative charge in the ion. Anions are attracted to
positively charged particles or regions.
Negative ions play crucial roles in various natural phenomena and technological applications. In atmospheric chemistry, for example, chloride ions (Cl⁻) and sulfate ions (SO₄²⁻) contribute to the formation of aerosols and cloud droplets, influencing weather patterns and precipitation. Nitrate ions (NO₃⁻) are essential components of fertilizers, supporting plant growth and agricultural productivity.
Polyatomic Ions: Molecular Assemblies
Polyatomic ions represent clusters of atoms bonded together
through covalent or coordinate covalent bonds, carrying a net electrical
charge. These molecular assemblies exhibit distinct chemical properties and can
act as single entities in chemical reactions.
Polyatomic ions are ubiquitous in chemical compounds and biochemical processes, playing diverse roles in nature and industry. For instance, sulfate ions (SO₄²⁻) are essential components of minerals, acids, and detergents, while ammonium ions (NH₄⁺) serve as nitrogen sources in fertilizers. Carbonate ions (CO₃²⁻) play a critical role in buffering systems, regulating pH levels in biological systems.
Conclusion: Illuminating the World of Ions
In conclusion, ions represent fundamental units of matter
that exert profound influences on the chemical and physical properties of
substances. From positive ions driving cellular processes to negative ions
shaping atmospheric phenomena, and polyatomic ions serving as building blocks
of compounds, the realm of ions encompasses a vast array of interactions and
applications. By unraveling the mysteries of ions, we gain deeper insights into
the fundamental principles governing the behavior of matter and unlock new
avenues for scientific exploration and technological innovation.


0 Comments